- Aluminium [Al] – Element Details, History, Atomic Structure, Facts, Properties, Electronic Configuration, Atomic Spectrum, Uses.
- Aluminium
- History of Aluminium
- How to Locate Aluminium on Periodic Table
- Aluminium Facts
- Aluminium Atomic Structure and Orbital Properties
- Element Properties
- Atomic Structure of Aluminium
- Ground State Electronic Configuration of Aluminium - neutral Aluminium atom
- Atomic Spectrum of Aluminium
- Regulatory and Health - Health and Safety Parameters and Guidelines
- Aluminium Chemical Properties : Aluminium Ionization Energies and electron affinity
- Use of Aluminium
Aluminium [Al] – Element Details, History, Atomic Structure, Facts, Properties, Electronic Configuration, Atomic Spectrum, Uses.

Aluminium
Aluminium is the 13th Element of Periodic table with atomic number 13, atomic weight 26.981538 , symbol Al, has a Face Centered Cubic structure and Silver color. Aluminium is a post-transition metal element. Trivial name of Aluminium is triels, icosagens. Know everything about Aluminium Facts, Physical Properties, Chemical Properties, Electronic configuration, Atomic and Crystal Structure.

History of Aluminium
The element Aluminium was discovered by None in year 1825 in Denmark . Aluminium derived its name from alumina, a compound (originally aluminum)
Aluminium (or aluminum; see different endings) is a chemical element in the boron group with symbol Al and atomic number 13. It is a silvery-white, soft, nonmagnetic, ductile metal. Aluminium is the third most abundant element (after oxygen and silicon), and the most abundant metal, in the Earth’s crust.
How to Locate Aluminium on Periodic Table
Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 13 to find Aluminium on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Aluminium on periodic table look for cross section of group 13 and period 3 in the modern periodic table
Aluminium Facts
Aluminium Atomic Structure and Orbital Properties
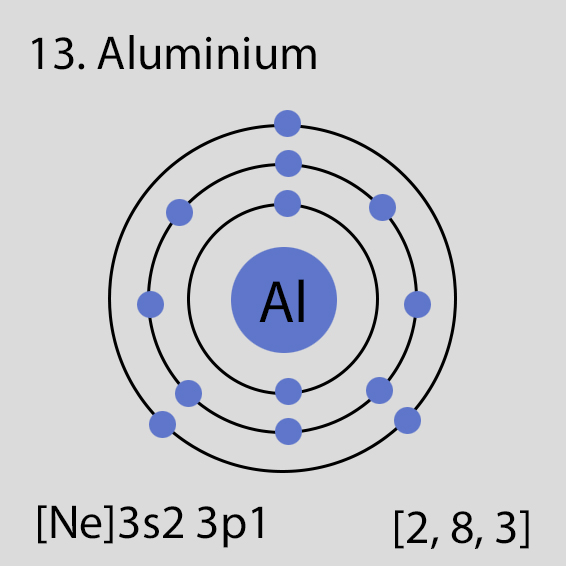
Aluminium atoms have 13 electrons and the electronic shell structure is [2, 8, 3] with Atomic Term Symbol (Quantum Numbers) 2P1/2.
Element Properties
2P1/2
Atomic Structure of Aluminium
Ground State Electronic Configuration of Aluminium - neutral Aluminium atom
The ground state electronic configuration of Neutral Aluminium atom is [Ne] 3s2 3p1. The portion of Aluminium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Ne]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.This is important as it is the Valence electrons 3s2 3p1, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Aluminium
Complete ground state electronic configuration for the Aluminium atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p1
Atomic Spectrum of Aluminium

Regulatory and Health - Health and Safety Parameters and Guidelines
Aluminium Chemical Properties : Aluminium Ionization Energies and electron affinity
Use of Aluminium
Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts. Since Aluminium is cheap and light weight, so it is also used in jewelry, everyday items, eyeglass frames, optical instruments, tableware etc.